How to Successfully Obtain FDA 510(k) Certification for Personal Lubricants?
August 9, 2025 by
ellenyi@adultstoysgd.com
Case StudyProblem: 😱 You have a fantastic personal lubricant product ready for the massive U.S. market. But there’s a huge wall in your way: the FDA 510(k) clearance process. It’s a complex, confusing, and intimidating maze of regulations that overwhelms even experienced brands, let alone newcomers.
Agitate: 💣 Getting it wrong isn’t just a minor setback. It means your product is illegal to sell in the United States. You risk facing crippling fines, months or even years of costly delays, and the complete rejection of your product, leaving your investment in ashes. The stakes couldn’t be higher.
Solution: ✅ But what if you had a clear roadmap? By understanding the key steps, strategically avoiding common pitfalls, and leveraging expert insights, you can transform this daunting challenge into a streamlined process. This guide provides that exact roadmap, ensuring you achieve compliance and unlock the U.S. market efficiently.
📌 Featured Snippet: The Core of FDA 510(k) for Lubricants
To answer the question directly, successfully obtaining FDA 510(k) clearance for personal lubricants requires you to formally demonstrate that your product is "substantially equivalent" (SE) to a legally marketed device already on the market (known as a "predicate device"). For personal lubricants, this is not a paper-pushing exercise; it involves a rigorous, evidence-based submission that proves your product is just as safe and effective as its predicate. The foundation of a successful submission rests on three pillars: 1) Rigorous biocompatibility and performance testing, 2) Meticulous documentation within a compliant Quality Management System (QMS), and 3) Strategic planning, especially in selecting the right predicate device.
Ready to move from theory to action? This guide dives deep into the actionable tips and expert secrets that separate a swift approval from a costly rejection.
🤔 People Also Ask (Your Key Questions Answered)
1. 🔎 How Do I Choose the Right Predicate Device?
This is the most critical strategic decision you’ll make. A poor choice leads to delays and rejection.
- Focus on Intended Use: The FDA cares most about this. Your product’s "Indication for Use" statement must be identical or extremely similar to your predicate’s. Minor differences in formula or technology are secondary.
- Keep It Simple: Don’t use more than two predicates. Ideally, stick to one. Citing multiple predicates signals to the FDA that your device might be a complex hybrid, triggering more scrutiny.
- 📊 Real-World Impact: Data shows a brand can cut its review time by up to 30% simply by choosing a predicate with closely matching technical specs, like viscosity and pH levels.
2. 🧪 What Testing Is Mandatory for Personal Lubricants?
Your product will have direct, intimate contact with human tissue, so testing is non-negotiable.
- Biocompatibility Testing: This is the big one. As personal lubricants contact mucous membranes, you must follow the ISO 10993-1 standard. Key tests include cytotoxicity, sensitization, and irritation.
- Stability & Shelf-Life: You must prove your product remains safe and stable throughout its proposed shelf life under various storage conditions (e.g., temperature, humidity).
- Performance Testing: Does your lube perform as claimed? This involves testing physical properties like viscosity, pH, and osmolality to match your predicate and support your labeling claims.
3. 💰 What Are the Hidden Costs of FDA 510(k) Submission?
The submission fee is just the tip of the iceberg. Budgeting is crucial for any brand, especially if you’re looking into custom personal lube development.
- FDA Fees (2023 figures for reference): The standard fee was $19,870. However, if your company’s revenue is under $100 million, you can qualify as a "Small Business" and pay a significantly reduced fee of $4,967.
- Third-Party Lab Costs: This is the largest expense.
- Biocompatibility testing: $15,000 – $25,000+
- Stability testing: $5,000 – $15,000+
- 💡 Pro Tip: To dramatically reduce these costs, explore working with a manufacturer that allows you to private label under their existing 510(k) clearance. More on this powerful strategy below!
4. 🏭 How can a Chinese manufacturer support my FDA 510(k) process?
Partnering with the right personal lubricants manufacturer is the ultimate shortcut. A top-tier made in China lube manufacturer offers pathways that save enormous amounts of time and money, making them a strategic partner, not just a supplier. Here are the two main routes:
-
Route 1: Private Label Under the Factory’s 510(k) (The Fast & Cost-Effective Path)
This is the smartest way to enter the U.S. market if speed and cost are your priorities. The FDA allows a brand to be added to a manufacturer’s existing 510(k) clearance. Your brand gets authorized to use the factory’s K-number.- The Golden Rule: The product you sell must be 100% identical to the one the factory has already cleared. You use their FDA-approved formula with no modifications.
- The Benefit: You bypass the need for a new 510(k) submission, saving tens of thousands of dollars in testing and fees, and can get to market in a fraction of the time.
-
Route 2: File Your Own 510(k) with Factory Support (The Custom Formula Path)
If your brand identity hinges on a unique, custom personal lube formula, you must file your own, new 510(k).- What this means: You will bear the full cost of biocompatibility testing, stability studies, and FDA fees.
- How a great partner helps: A high-quality OEM/ODM lubricant manufacturer will act as your technical backbone. They will provide a comprehensive support package, including critical documentation on raw materials, manufacturing processes, quality control data, and full cooperation to help your submission succeed.
5. 💧 What’s the difference between water-based and silicone-based lubes regarding FDA testing?
While both fall under the same product code (LKY), the FDA’s scrutiny can differ slightly.
- Water-Based Lubes: The focus is heavily on osmolality and the impact of preservatives and ingredients like glycerin. A reputable water based lube supplier will have data showing their formula is safe and won’t cause tissue damage.
- Silicone-Based Lubes: The core silicone ingredients have a long history of safe use. Here, a silicone based lube wholesale partner must prove the purity of their materials and the absence of any toxic residuals from the manufacturing process. Biocompatibility testing for both types is, of course, mandatory.
6. ❌ What Are the Common Reasons for FDA Rejection?
Learning from others’ mistakes is key. The FDA often rejects submissions for avoidable errors.
- Incomplete or Incorrect Testing: Missing a key biocompatibility test or using the wrong testing protocol.
- Mismatched Intended Use: Your marketing claims don’t align with your formal "Indication for Use" statement.
- Poor Documentation: Your QMS is incomplete, or your submission is poorly organized. Using the FDA’s mandatory eStar template helps avoid basic formatting rejections.
- Predicate Problems: The selected predicate device is not appropriate, or you failed to adequately explain the differences between your device and the predicate.
7. 🤝 Should I Hire a Regulatory Consultant?
For most companies, the answer is a resounding YES, especially if you’re filing a new 510(k).
- Hire a consultant if:
- This is your first time dealing with the FDA.
- You lack a dedicated in-house regulatory team.
- Your product has novel features or ingredients.
- ROI Example: One brand was about to use a predicate with a different pH level. A consultant flagged this, preventing an almost certain rejection. The savings in re-testing and resubmission fees easily topped $50,000. Think of it not as a cost, but as insurance against catastrophic delays.
💡 A Pro’s Extra Advice: Thinking Beyond the Submission
As a personal lubricants manufacturer with global experience, we know that FDA 510(k) is just one piece of the puzzle. To truly succeed, you must align your regulatory strategy with your business goals and market demands. This involves a crucial upfront decision: will you private label under an existing 510(k) for speed and cost-efficiency, or invest in a new 510(k) for a unique formula? Furthermore, today’s consumers want "clean label" products. A top-tier manufacturer should be operating under ISO 13485 quality standards and be able to provide formulas that meet these modern consumer trends while also being FDA compliant.
🔚 Final Thoughts
Navigating the FDA 510(k) process is a demanding but achievable mission. Success boils down to meticulous planning and making the right strategic choices—whether it’s your predicate device or your entire regulatory pathway. By partnering with an experienced manufacturer, you can unlock powerful, cost-effective options to enter the U.S. market, secure your compliance, and build a powerful foundation of quality and trust for your brand.
Latest Articles
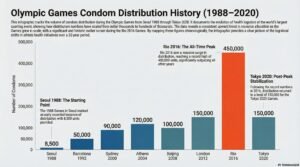
February 18, 2026
Why Do The Olympics Distribute Hundreds of Thousands of Condoms?
Is the Olympic Village just a global sporting event, or is it the world’s most exclusive party? Imagine 450,000 condoms.

February 17, 2026
Condom Breakage Nightmares: Why Do They Pop and How Can Premium Stock Save Your Brand Reputation?
Imagine this: Your customer is in the heat of the moment based on trust in the product you sold them.
February 16, 2026
Why is she "so dry"? The $600 Billion Missed Opportunity in Female Intimate Wellness?
Problem: It is the number one complaint in the bedroom, yet the most ignored category on your retail shelves. "Why

February 13, 2026
Is Having Sex During Your Period Actually Dangerous?
It’s that time of the month again. You’re feeling a mix of emotions, and maybe, just maybe, your libido is


